- Global Audit Library
- Services
Our comprehensive support for full compliance of medical devices, offering you the peace of mind you deserve. + More
- About Us
- Resource Hub
- Login
- Contact Us
Our comprehensive support for full compliance of medical devices, offering you the peace of mind you deserve. + More
However, the FDA’s ‘risk-based-approach’ initiative and, later, the guidelines that emerged from that initiative: ICH Q8, Q9, Q10, Process Analytical Technology (PAT)… brought the requirement for the application of GxP principles (GLP, GMP, GCP) to the entire Lifecycle of a pharmaceutical product in a much higher level.
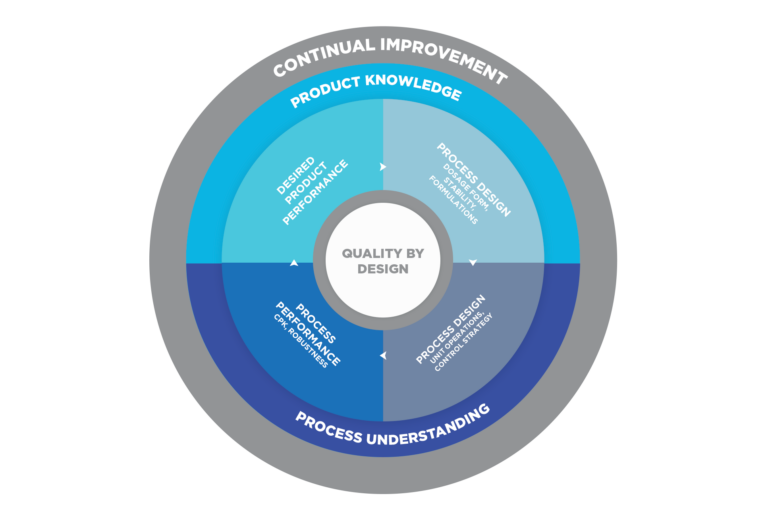
Concepts such as Quality by Design (QbD) are applied since the initial stages of development. The application of solid scientific criteria for the evaluation of results and data or the design of experiments, contribute to the knowledge and understanding of the product and the process, which is the key for obtaining and demonstrating the necessary control on the process and its design and the consistent quality of the product.
During the development phases of drugs, products used in clinical trials must be manufactured with quality standards equivalent to those applied in commercial drugs, adapting some requirements to the development phases. The labelling and secondary packaging of clinical trial medication is an essential part of the process to ensure that volunteers or designated patients take assigned medication..



We provide extensive GMP consulting services to help keep our clients ahead of the needs and expectations of regulators.
Explore our extensive GMP audit library to see the range and scope of live reports we have in stock, join a live audit, or commission a bespoke audit
Maintain high standards of life sciences manufacturing supplier qualifications and GMP auditing within the supply chain through our expertise
Discover how we can help your product reach to market, fully and demonstrably complying with the latest GxP standards
From data integrity to implementing new systems, our experienced team with a digital mindset, can lead you to transformative achievements
REPHINE CHINA
REPHINE INDIA
Sign up to our newsletter to get the latest news about Rephine and industry news.